
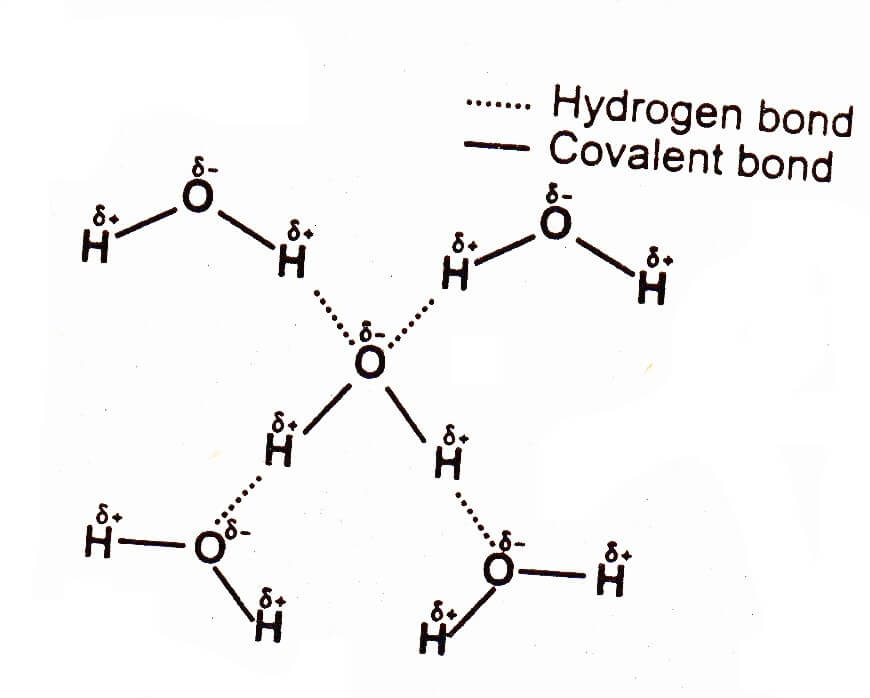
Now scientists don't like writing stuff that means it's easy to understand. If it's slightly negative that means this hydrogen here is slightly positive. That makes this oxygen slightly negative. So what that means is that these negative electrons tend to spend a little bit more time around the oxygen than around the hydrogen.

They are essentially having only visitational rights on the weekend as opposed to equal co-rights. So these electrons spend most of their time orbiting around the oxygen, very little time orbiting around the hydrogen. So it tends to pull the electrons much closer to it and further away from the hydrogen which is too weak to hold on to these electrons very well. Oxygen on the other hand is electro negative strong one. The two electrons here that form this bond are shared equally between the carbon and the hydrogen, same over here. It has roughly the same electro negativity as the hydrogens that are bound to it, indicated by these black lines because as roughly the same electro negativity. Now carbon here I see a methane molecule CH4. In short electro negativity is essentially how strongly does that atom pull on its electrons. Now how does this work? Well, it's all based on this quality that atoms have called electro negativity. You may not really get that cause you know what ionic and covalent bonds are but how does that fit into this? Hydrogen bonds are a weird category of bonds all kind of by themselves because unlike ionic or covalent bonds which hold individual atoms together to form molecules, hydrogen bonds are typically weak attractions between two different molecules that hold those two different molecules close to each other. Well you may have read in textbooks this kind of definition for hydrogen bonds that the weak attractions between partially positive hydrogens and partially negative oxygens or nitrogens.


 0 kommentar(er)
0 kommentar(er)
